
Colours
Colours can change your mood, take movies for instance. A dark, grey scene indicates mourning and the absense of bright colours indicates a dreadful routine like a boring office job.
And removing 'green' entirely you get the openings scene of Wall-E, leaving a desolate and baren feeling. Almost dystopian.
To fully understand colours, a.k.a. coloured light, we need to understand light first. So, what is light exactly and how can light be used to laser in colour on stainless steel?
Light
Light is fascinating and has stunned scientists for years. While Isaac Newton proved light is a particle, at the same time Christiaan Huygens proved light was a wave, like a soundwave. Which shouldn't be possible, at least not at the same time.
To laser-mark a colour we use the wave-part of light. If you've been to the coast or stood in the surf you know that 2 waves colliding can make a bigger wave, or cancel each other out. Noise-cancelling headphones use this principle too, but with sound-waves.
Graph A shows making the bigger wave (constructive interference), graph B the 'noise-cancelling' (destructive interference).
And light waves just so, making it twice as bright or dark. The official term is interference and seems weird when you think about it. When you shine 2 lasers extremely precise you get... Darkness, basically nothing.


Light-waves and Stainless Steel
Light of a specific colour has a certain wavelength, just as a music-tone. This, together with the interference-properties of waves is what is needed to laser-mark in colour.
Stainless steel has a thin oxide layer that prevents corrotion from propegating through the metal. But molten stainless steel will oxidize happily and does so. A proper laser can melt a very precize layer between 0,0002mm to 0,00035mm, controlling the thickness of the oxide layer in the nanometer-range.
That oxide layer is semi-transparant, meaning that part of the lightrays hitting it will reflect off like a mirror, the other part continous on as the oxide layer is a window. But it will hit the non-oxidized layer behind it, which is acting like a full mirror.
The final clue
The light rays that are reflected off the oxide layer have travelled less distance than those that went through it and reflected of the stainless steel. If the thickness of the oxide layer is the same of the wavelength, 2 lightwaves will meet each other, collide and form a bigger wave. Since both lightrays are the same it is safe to assume this wave gets twice as big.
A bigger lightwave is what humans perceive as brighter. Hence, by changing the thickness of the oxide layer we can choose which colour reflects twice as bright and 'overpowers' the other colours.

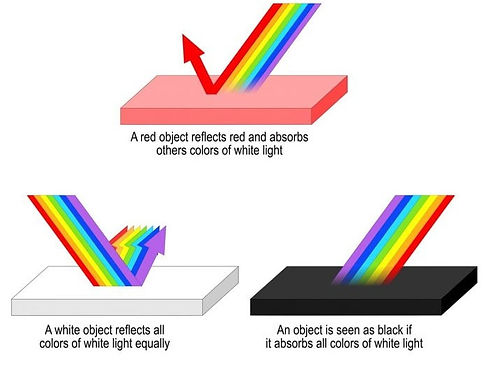
Normal colours
Normal colours are made differently. Normal white light is a giant mess of (literally) all the colours of the rainbow going in all directions. A colour is only a colour because it absorbs all colours, except the one that it is. That colour is reflected and will eventually hit our eye and we see the object on only that colour.
White is white because it reflects all colours and absorbs none. If you'd polish a white surface you get a mirror.
And black? Technically darkness does not excist, it is only an absense of light. Hence it absorbs all lightrays and reflects none.
This absorption and reflection can be greatly improved by a laser-polishing or roughening as well.
Nature's colour
Nature is full of colours and all have a function, possibly several. Making a pigment from food cost energy that is better spend on surviving and reproducing.
Most pigments are hard to make, blue is even impossible. The solution is the same as described here above, by interference. It is striking that a simple butterfly uses physics that we hardly understand.
The peacock-butterfly was found on WikiMedia (Click here) where was zoomed all the way in, bottom right, where you could see nanometer-sized features, 0,000001 mm. Those small sliths are responsible for the colour, a different size would result in a different colour.
Besides butterflies there are many other species using this technique. Many beetles have a blue-purpleish shield, a peacock, even male ducks have a but of blue on their wings.
The main key in identifying if a colour is iridescent is the glimmering in the sunlight and for animals the colour blue. Any blue in any animal is 100% 'fake'.


Rabbit hole
Below links to more information if you want to continue wandering through the forest of knowledge. I'll leave you here and I'm sure that you'll see colours, or blue eyes for that matter, in a whole different light!
P.S. the top-peacock photo is taken at dawn, the bottom at noon. Now you can clearly see which colours are iridescent and that a peacock is actually brown/greyish.
Colour usages in film:
https://www.youtube.com/watch?v=tILIeNjbH1E
Structural coloration
https://en.wikipedia.org/wiki/Structural_coloration
Iridescence
https://en.wikipedia.org/wiki/Iridescence
Light is a wave and a particle
https://www.youtube.com/watch?v=Iuv6hY6zsd0
Light can travel back through time